Health
B.C. Review Urges Changes in Rare-Disease Drug Funding System
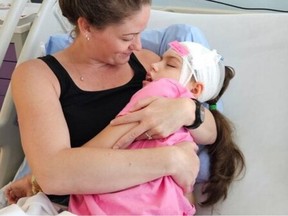
A review conducted by the British Columbia (B.C.) government four years ago recommended significant changes to the funding and treatment of rare diseases, focusing on transparency, communication, and decision-making processes. This comes in light of recent calls from Premier David Eby for reforms to the current system, particularly after public outcry regarding the discontinuation of a costly drug treatment for a young patient.
The leaked report, which surfaced independently through Postmedia, highlighted the urgency of addressing the growing financial burden associated with rare-disease drugs. Spending on these medications is projected to reach an estimated $600 million annually by the end of the decade. Despite the report’s findings, many of its recommendations remain unimplemented, as indicated by experts familiar with its contents.
Dean Regier, an associate professor at the University of British Columbia (UBC), remarked, “The recommendations are still relevant. This is going to be an increasing challenge for health systems to navigate expensive drugs for rare diseases.” His observations underscore the pressing need for effective strategies to manage the complexities of funding and access to these medications.
In response to inquiries, the B.C. Ministry of Health stated that the majority of the report’s recommendations have been enacted or are part of ongoing initiatives. However, the ministry only cited two completed actions from over 300 recommendations: the creation of a dedicated web page for rare disease drugs and the establishment of an appeal process. Critics note that the recommendations for enhanced transparency extend beyond a website, encompassing a comprehensive communication plan, increased oversight, and public engagement strategies.
The report was delivered to the NDP government in spring 2021 and aligns closely with Eby and Health Minister Josie Osborne‘s recent statements regarding the need for systemic reform. Their government faced backlash after discontinuing an $800,000 annual treatment for Charleigh Pollock, a ten-year-old suffering from a degenerative disease. Following public pressure, the government reversed its decision last month, which led to the resignation of ten members from its advisory committee.
The existence of the four-year-old review, which was not made public, raises questions about the government’s commitment to transparency and accountability. Neither Eby nor Osborne referenced the recommendations during their recent discussions about necessary changes to the system. The Health Ministry confirmed that staff are currently verifying which recommendations have been implemented, with plans for further assessments as part of an ongoing review.
One advisory committee member who resigned, Dr. Sandra Sirrs, expressed her support for increased transparency regarding expensive rare disease drugs. She emphasized that the public’s response to recent events indicates that previous measures, such as the website initiative, have not sufficed. She stated, “Had these steps been taken, people in British Columbia could have been better equipped to understand the complex issues in the recent B.C. case that has dominated the media.”
Regier noted that engaging the public and gathering feedback on how to allocate limited health resources remain critical components of any successful reform. He stressed the importance of developing a system to collect real-world data on expensive drugs, which often lack clear evidence of effectiveness, and establishing mechanisms for discontinuing coverage when necessary.
Last year, B.C. allocated $200 million for rare disease drugs, serving approximately 600 patients. The report’s recommendations encompass a range of strategies for public funding decisions, including strengthening oversight and establishing evidence standards for drug effectiveness. Some of these recommendations require collaboration with federal authorities to be fully realized.
The review included input from various stakeholders, including representatives from the B.C. Ministry of Health, the Provincial Health Services Authority, and the Drug Benefit Council, which provides evidence-based recommendations to the Ministry regarding drug listings. The report highlighted the need for transparent decision-making processes, stating, “Dealing with high emotion and high publicity challenges will require courageous leadership.”
It also warned that taxpayers might question the rationale behind funding expensive drugs for a limited number of individuals through their taxes. The report called for the establishment of an oversight body to manage the system for rare disease drugs in collaboration with the Provincial Health Services Authority.
The communication strategy recommended in the report aimed to ensure transparency and build public trust. It emphasized the need to engage the public in a meaningful way, facilitating informed feedback that could guide decisions on drug funding. The strategy proposed proactive media engagement to address controversial decisions and a public relations framework that accurately reflects the complexities of government decision-making regarding healthcare funding.
Recently, Eby reiterated the need for reforms to improve transparency and enhance public understanding of the decision-making process related to rare disease drugs. Osborne echoed these sentiments, suggesting that greater transparency will help build trust and ensure that patients and families feel heard throughout the decision-making process.
As the B.C. government navigates the challenges of rare-disease drug funding, the recommendations from the four-year-old review serve as a critical reminder of the complexities involved and the urgent need for comprehensive reform. The long-term implications for both patients and the healthcare system depend on effective implementation of these strategies to ensure fair access to necessary treatments.
-

 Science1 week ago
Science1 week agoMicrosoft Confirms U.S. Law Overrules Canadian Data Sovereignty
-

 Technology1 week ago
Technology1 week agoGoogle Pixel 10 Pro Fold Specs Unveiled Ahead of Launch
-

 Technology1 week ago
Technology1 week agoWorld of Warcraft Players Buzz Over 19-Quest Bee Challenge
-

 Science1 week ago
Science1 week agoXi Labs Innovates with New AI Operating System Set for 2025 Launch
-

 Science5 days ago
Science5 days agoChina’s Wukong Spacesuit Sets New Standard for AI in Space
-

 Health5 days ago
Health5 days agoRideau LRT Station Closed Following Fatal Cardiac Incident
-

 Technology1 week ago
Technology1 week agoHumanoid Robots Compete in Hilarious Debut Games in Beijing
-

 Science1 week ago
Science1 week agoInfrastructure Overhaul Drives AI Integration at JPMorgan Chase
-

 Top Stories1 week ago
Top Stories1 week agoSurrey Ends Horse Racing at Fraser Downs for Major Redevelopment
-

 Lifestyle5 days ago
Lifestyle5 days agoVancouver’s Mini Mini Market Showcases Young Creatives
-

 Technology1 week ago
Technology1 week agoNew IDR01 Smart Ring Offers Advanced Sports Tracking for $169
-

 Technology1 week ago
Technology1 week agoFuture Entertainment Launches DDoD with Gameplay Trailer Showcase
-

 Science1 week ago
Science1 week agoNew Precision Approach to Treating Depression Tailors Care to Patients
-

 Technology1 week ago
Technology1 week agoGlobal Launch of Ragnarok M: Classic Set for September 3, 2025
-

 Technology1 week ago
Technology1 week agoInnovative 140W GaN Travel Adapter Combines Power and Convenience
-

 Business1 week ago
Business1 week agoNew Estimates Reveal ChatGPT-5 Energy Use Could Soar
-

 Technology5 days ago
Technology5 days agoDragon Ball: Sparking! Zero Launching on Switch and Switch 2 This November
-

 Health1 week ago
Health1 week agoGiant Boba and Unique Treats Take Center Stage at Ottawa’s Newest Bubble Tea Shop
-

 Business5 days ago
Business5 days agoCanadian Stock Index Rises Slightly Amid Mixed U.S. Markets
-

 Lifestyle1 week ago
Lifestyle1 week agoEleven Madison Park to Reinstate Meat After Vegan Experiment
-

 Business1 week ago
Business1 week agoSimons Plans Toronto Expansion as Retail Sector Shows Resilience
-

 Business1 week ago
Business1 week agoUkraine Strikes Lukoil Refinery, Halting Operations Amid Conflict
-

 Science1 week ago
Science1 week agoNew Study Reveals Surprising Impact of Gratitude on Helping Behaviors
-

 Lifestyle4 days ago
Lifestyle4 days agoOntario Woman Wins $100,000 Lottery Prize from Brother’s Gift










