

In 1923, inventor Leo Gerstenzang created Q-tips after observing his wife use cotton on toothpicks for various tasks. Initially designed for baby care and other household...



Australian biotechnology firm Mesoblast has announced a significant increase in sales of its cell therapy, Ryoncil, which achieved gross revenue of $35.1 million during the December...



Sanofi’s Tzield has been accepted for priority review by the U.S. Food and Drug Administration (FDA) for use in young children diagnosed with stage 2 type...
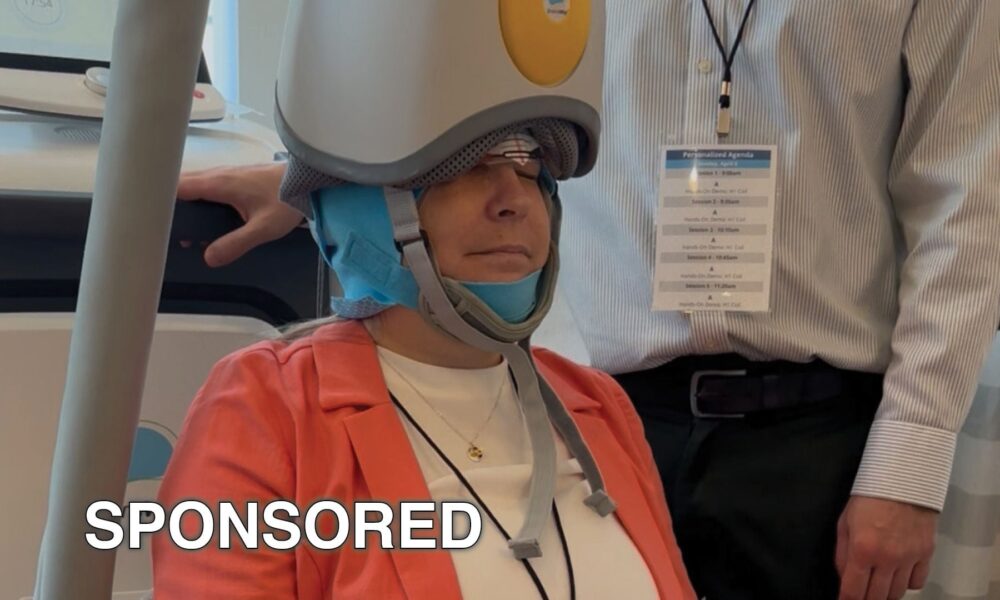


The Braden Center in Sterling, Illinois, has introduced BrainsWay’s Deep TMS (Deep Transcranial Magnetic Stimulation), a significant advancement in the treatment of treatment-resistant depression (TRD). This...



Incyte Corporation announced on October 29, 2023, that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation to its investigational drug, INCA033989. This...



The U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation to Incyte’s experimental drug, INCA033989, aimed at treating a rare blood cancer known as...



The U.S. Food and Drug Administration (FDA) has officially extended the review period for the New Drug Application (NDA) concerning Ascendis Pharma’s treatment for children with...



The U.S. Food and Drug Administration (FDA) has initiated a trial program aimed at improving communication with pharmaceutical companies regarding pending drug applications. Launched in October...
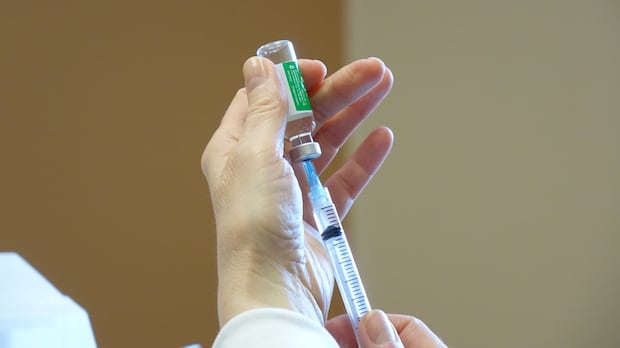
A recent study from the University of Waterloo has provided valuable insights into the effectiveness of COVID-19 vaccines against emerging strains of the virus. Conducted by...



The U.S. Food and Drug Administration (FDA) has announced a significant recall of more than 580,000 bottles of the blood pressure medication prazosin hydrochloride. This action...