Health
B.C. Review Urged Changes to Rare-Disease Drug System Four Years Ago
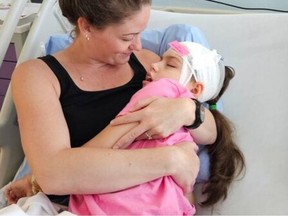
A leaked report has revealed that a review conducted by the British Columbia (B.C.) government four years ago recommended significant changes to the province’s approach to funding and treating rare diseases that require expensive drugs. This review, which has not been publicly disclosed until now, emphasized the need for improved transparency, clearer communication, and stronger decision-making processes in the healthcare system.
The findings of the report, which were delivered to the NDP government in the spring of 2021, highlighted an urgent need for reform. By the end of the current decade, spending on rare-disease drugs was projected to reach approximately $600 million annually. Yet, according to sources familiar with the report, few of its recommendations have been acted upon.
Dean Regier, an associate professor at the University of British Columbia’s School of Population and Public Health, noted the ongoing relevance of the report’s recommendations. He stated, “It’s quite apparent over the last decade or so that this is going to be an increasing challenge for health systems to navigate expensive drugs for rare diseases, including for safety, efficacy, cost-effectiveness, and the sustainability of our healthcare system.”
In a written statement, the B.C. Ministry of Health claimed that the “majority” of the report’s recommendations have either been implemented or are part of ongoing initiatives. However, officials pointed to only two completed items out of more than 300 recommendations: the creation of a website dedicated to rare-disease drugs for enhanced transparency and the establishment of an appeal process for drug funding decisions.
The recommendations related to transparency are extensive, with 39 specific mentions in the report. These suggestions extend beyond just creating a website; they call for increased oversight, the establishment of standards, public engagement, and a comprehensive communication strategy.
The recent remarks from Premier David Eby and Health Minister Josie Osborne indicate they are now prioritizing these issues, particularly after facing public backlash for discontinuing a costly $800,000 annual treatment for 10-year-old Charleigh Pollock, who suffers from an incurable degenerative disease. After public outcry, the government reversed its decision last month, a move that contradicted the advice of its own 58-member advisory committee and led to resignations from ten committee members.
The previously confidential review was obtained by Postmedia independently after the B.C. government declined to release it. Notably, neither Eby nor Osborne has referenced the four-year-old recommendations in their recent calls for reform.
The Health Ministry stated that staff are currently verifying the status of the recommendations and will assess remaining ones as part of an ongoing review. Health Minister Osborne was unavailable for an interview.
Dr. Sandra Sirrs, an advisory committee member who resigned, expressed her support for increased transparency in the drug approval process. She emphasized that many unimplemented recommendations from the 2021 report focused on public education and awareness about the complexities of rare-disease treatments. “Had these steps been taken, people in British Columbia could have been better equipped to understand the complex issues in the recent B.C. case which has dominated the media,” she stated.
Regier, who was also involved in the review, highlighted the importance of engaging the public in understanding the challenges and obtaining feedback on resource allocation for public health. He noted the necessity of collecting real-world data on these expensive drugs, which often lack clear evidence of effectiveness, and establishing mechanisms to discontinue coverage for ineffective treatments.
Last year, B.C. spent approximately $200 million on rare-disease drugs for around 600 patients. The review’s 43-page report provided detailed recommendations on how to improve the decision-making process regarding public funding for rare-disease drugs and how to enhance the system for determining which drugs are approved both provincially and nationally.
Many of the recommendations require cooperation from federal health agencies, as highlighted by B.C. Health Ministry officials. The review involved input from a range of stakeholders, including representatives from the B.C. Ministry of Health, the Provincial Health Services Authority, and the Drug Benefit Council, which advises the Ministry on drug listings for pharmacare.
The report underscored the need for courageous leadership and a well-justified decision-making foundation in the face of high-stakes and emotionally charged challenges. It warned that taxpayers may eventually question the rationale behind funding high-cost medications for a limited number of individuals through their taxes.
One of the key recommendations was to establish a dedicated body to oversee the implementation of the rare-disease drug system, ensuring collaboration with the Provincial Health Services Authority. The report also called for setting evidence standards and effectiveness thresholds for rare-disease drugs and integrating rare-disease patients into the pharmacare program.
Health Ministry officials indicated that the decision was made not to add rare-disease patients to pharmacare coverage. Additionally, the report included a comprehensive communication and engagement strategy, emphasizing the importance of transparency regarding decision criteria for healthcare partners, including healthcare professionals and the public.
Premier Eby has recently stated the need to enhance transparency within the rare-disease drug system to better serve the public. He underscored the importance of ensuring that decisions are made by experts and that the processes are comprehensible for those affected. Osborne added that “greater transparency can help build trust and ensure that patients and families feel heard and informed as decisions are made.”
-

 Science1 week ago
Science1 week agoMicrosoft Confirms U.S. Law Overrules Canadian Data Sovereignty
-

 Technology1 week ago
Technology1 week agoGoogle Pixel 10 Pro Fold Specs Unveiled Ahead of Launch
-

 Technology1 week ago
Technology1 week agoWorld of Warcraft Players Buzz Over 19-Quest Bee Challenge
-

 Science5 days ago
Science5 days agoChina’s Wukong Spacesuit Sets New Standard for AI in Space
-

 Health6 days ago
Health6 days agoRideau LRT Station Closed Following Fatal Cardiac Incident
-

 Science1 week ago
Science1 week agoXi Labs Innovates with New AI Operating System Set for 2025 Launch
-

 Lifestyle6 days ago
Lifestyle6 days agoVancouver’s Mini Mini Market Showcases Young Creatives
-

 Science1 week ago
Science1 week agoInfrastructure Overhaul Drives AI Integration at JPMorgan Chase
-

 Technology1 week ago
Technology1 week agoHumanoid Robots Compete in Hilarious Debut Games in Beijing
-

 Top Stories1 week ago
Top Stories1 week agoSurrey Ends Horse Racing at Fraser Downs for Major Redevelopment
-

 Technology1 week ago
Technology1 week agoNew IDR01 Smart Ring Offers Advanced Sports Tracking for $169
-

 Technology5 days ago
Technology5 days agoDragon Ball: Sparking! Zero Launching on Switch and Switch 2 This November
-

 Business6 days ago
Business6 days agoCanadian Stock Index Rises Slightly Amid Mixed U.S. Markets
-
 Health6 days ago
Health6 days agoB.C. Review Urges Changes in Rare-Disease Drug Funding System
-

 Science1 week ago
Science1 week agoNew Precision Approach to Treating Depression Tailors Care to Patients
-

 Technology1 week ago
Technology1 week agoGlobal Launch of Ragnarok M: Classic Set for September 3, 2025
-

 Technology1 week ago
Technology1 week agoFuture Entertainment Launches DDoD with Gameplay Trailer Showcase
-

 Education5 days ago
Education5 days agoParents Demand a Voice in Winnipeg’s Curriculum Changes
-

 Technology1 week ago
Technology1 week agoInnovative 140W GaN Travel Adapter Combines Power and Convenience
-

 Business1 week ago
Business1 week agoNew Estimates Reveal ChatGPT-5 Energy Use Could Soar
-

 Health5 days ago
Health5 days agoRideau LRT Station Closed Following Fatal Cardiac Arrest Incident
-

 Business5 days ago
Business5 days agoAir Canada and Flight Attendants Resume Negotiations Amid Ongoing Strike
-

 Health1 week ago
Health1 week agoGiant Boba and Unique Treats Take Center Stage at Ottawa’s Newest Bubble Tea Shop
-

 Business1 week ago
Business1 week agoSimons Plans Toronto Expansion as Retail Sector Shows Resilience










