Science
Trump Extends Ethylene Oxide Compliance Deadline for Sterilization
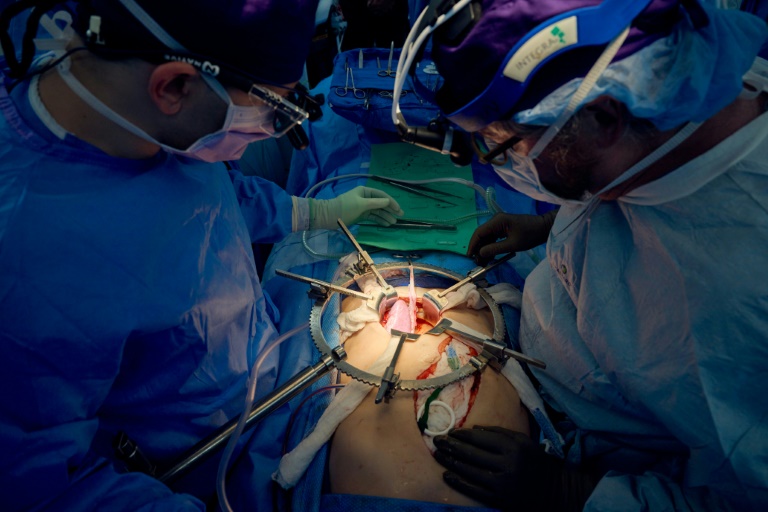
President Donald Trump has issued an executive order extending the compliance deadline for medical device sterilization facilities regarding the use of ethylene oxide (EtO) by two years. This decision impacts 39 facilities specifically named in the order and allows them additional time to meet new emissions standards set by the U.S. Environmental Protection Agency (EPA) in March 2024.
Ethylene oxide is a key agent used to sterilize medical devices, ensuring they are free from harmful microorganisms. Concerns surrounding its use stem from its mutagenic properties, which can lead to DNA mutations and increase cancer risk. The recent reversal raises eyebrows, particularly as regulatory efforts had aimed to significantly reduce EtO emissions, with the EPA’s final rule designed to cut these emissions by over 90% through the installation of advanced air pollution controls.
Regulatory Background and Recent Changes
The executive order reflects a shift in the U.S. government’s stance on emissions-control technology, with the administration claiming that the required technology is not commercially viable. This claim is particularly contentious, given that international standards, such as ISO 10993-7 and ISO 11135, already govern the use of EtO in medical device sterilization. These standards set strict limits on residual EtO levels, aligning with global health and safety concerns.
The EPA’s March 2024 rule affects nearly 90 commercial sterilization facilities operated by about 50 companies across the country. The new regulations established requirements for building leaks, chamber exhaust vents, and mandated continuous emissions monitoring to ensure compliance. Under the previous standard, approximately 50% of all sterile medical devices in the U.S. were sterilized using ethylene oxide, a method that remains essential for many products that cannot withstand alternative sterilization processes.
Implications for the Medical Device Industry
Trade groups representing the medical device industry had been vocal in their lobbying efforts for a delay, arguing that the transition to new technologies posed significant logistical and economic challenges. These groups raised concerns about potential supply chain disruptions, emphasizing that the compliance deadlines were unrealistic given the current state of technology.
The executive order invokes section 112(i)(4) of the Clean Air Act, which grants the President the authority to provide compliance exemptions under specific circumstances. Trump’s order specifies that the technology necessary for compliance does not exist in a commercially viable form and that the exemption serves the national security interests of the United States.
The decision has drawn criticism from health advocates and environmental groups, who argue that extending the compliance deadline undermines public health efforts. Ethylene oxide is known to cause a range of health issues, including respiratory irritation, headaches, and severe long-term effects like lung damage and heart problems upon exposure.
In Europe, the use of ethylene oxide has been declining, primarily due to stringent regulatory measures aimed at protecting public health. The UK’s medicines regulator has consistently expressed concerns about residual EtO levels and their potential risks to human health, favoring alternatives like gamma radiation for sterilization.
The implications of this reversal are significant, as the medical device industry navigates the balance between maintaining supply chains and addressing health risks associated with ethylene oxide. The extended timeline may provide temporary relief, but it also raises questions about the future direction of sterilization practices within the industry.
As the landscape of medical device sterilization evolves, the ongoing debate over the safety and efficacy of ethylene oxide continues to be a pivotal issue in public health and environmental policy.
-

 Education3 months ago
Education3 months agoBrandon University’s Failed $5 Million Project Sparks Oversight Review
-

 Science4 months ago
Science4 months agoMicrosoft Confirms U.S. Law Overrules Canadian Data Sovereignty
-

 Lifestyle3 months ago
Lifestyle3 months agoWinnipeg Celebrates Culinary Creativity During Le Burger Week 2025
-

 Health4 months ago
Health4 months agoMontreal’s Groupe Marcelle Leads Canadian Cosmetic Industry Growth
-

 Technology3 months ago
Technology3 months agoDragon Ball: Sparking! Zero Launching on Switch and Switch 2 This November
-

 Science4 months ago
Science4 months agoTech Innovator Amandipp Singh Transforms Hiring for Disabled
-

 Education3 months ago
Education3 months agoRed River College Launches New Programs to Address Industry Needs
-

 Technology4 months ago
Technology4 months agoGoogle Pixel 10 Pro Fold Specs Unveiled Ahead of Launch
-

 Business3 months ago
Business3 months agoRocket Lab Reports Strong Q2 2025 Revenue Growth and Future Plans
-

 Technology2 months ago
Technology2 months agoDiscord Faces Serious Security Breach Affecting Millions
-

 Education3 months ago
Education3 months agoAlberta Teachers’ Strike: Potential Impacts on Students and Families
-
 Science3 months ago
Science3 months agoChina’s Wukong Spacesuit Sets New Standard for AI in Space
-

 Education3 months ago
Education3 months agoNew SĆIȺNEW̱ SṮEȽIṮḴEȽ Elementary Opens in Langford for 2025/2026 Year
-

 Technology4 months ago
Technology4 months agoWorld of Warcraft Players Buzz Over 19-Quest Bee Challenge
-

 Business4 months ago
Business4 months agoNew Estimates Reveal ChatGPT-5 Energy Use Could Soar
-

 Business3 months ago
Business3 months agoDawson City Residents Rally Around Buy Canadian Movement
-
 Technology2 months ago
Technology2 months agoHuawei MatePad 12X Redefines Tablet Experience for Professionals
-

 Business3 months ago
Business3 months agoBNA Brewing to Open New Bowling Alley in Downtown Penticton
-

 Technology4 months ago
Technology4 months agoFuture Entertainment Launches DDoD with Gameplay Trailer Showcase
-

 Technology4 months ago
Technology4 months agoGlobal Launch of Ragnarok M: Classic Set for September 3, 2025
-

 Technology4 months ago
Technology4 months agoInnovative 140W GaN Travel Adapter Combines Power and Convenience
-

 Science4 months ago
Science4 months agoXi Labs Innovates with New AI Operating System Set for 2025 Launch
-

 Top Stories2 months ago
Top Stories2 months agoBlue Jays Shift José Berríos to Bullpen Ahead of Playoffs
-

 Technology4 months ago
Technology4 months agoNew IDR01 Smart Ring Offers Advanced Sports Tracking for $169










