Science
Montreal Device Uses AI to Revolutionize Cancer Surgery and Save Lives
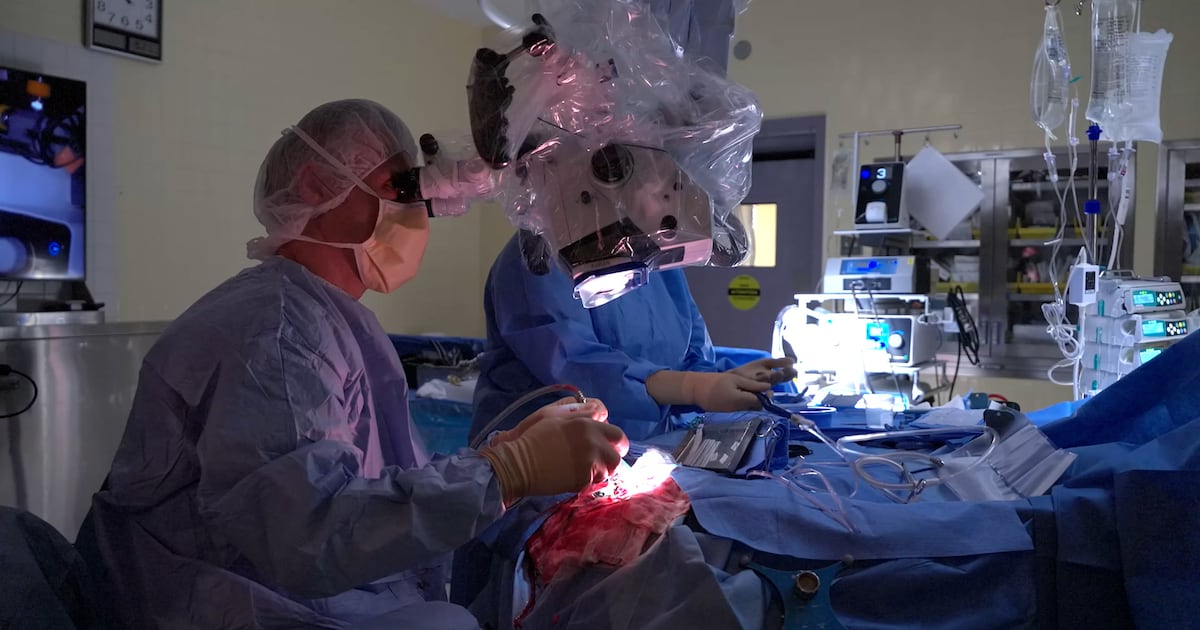
A groundbreaking surgical device developed in Montreal is transforming cancer treatment by using artificial intelligence (AI) to significantly improve patient outcomes. The device, known as SENTRY, assists surgeons in distinguishing between cancerous and healthy tissue, offering hope to patients like Peter Ross, who was given just 14 months to live after being diagnosed with a brain tumor nearly four years ago.
Ross, now preparing to become a grandfather, attributes his extended life to this innovative technology. “It’s given me the rest of my life,” he expressed, reflecting on his journey. His wife, Sandrine Menard, echoed his sentiment, stating, “Whatever happens, we’re grateful.”
How SENTRY Works
The SENTRY device, co-founded by surgeon Dr. Kevin Petrecca at the Montreal Neuro, allows for real-time identification of tumor cells during surgery. According to Petrecca, “98.7 percent of the time, if it says it’s tumor, it’s tumor.” Even more crucially, when the device indicates normal brain tissue, it is correct 100 percent of the time.
This technology addresses a significant challenge in cancer surgeries, where tumors often remain undetectable to the naked eye and traditional imaging techniques. With SENTRY, surgeons can receive feedback in less than three seconds, enabling them to remove more cancerous tissue than previously possible. Petrecca noted that utilizing this device can potentially extend a patient’s life two to five times longer than expected.
While SENTRY has primarily been tested in brain surgeries, its applications extend to other cancers, including those affecting the breast and lungs. Hundreds of patients have participated in clinical trials, including Ross, who shared, “I take every extra day that’s given to me to go out and walk, and just enjoy life because I owe that to this surgery.”
Next Steps for SENTRY
As SENTRY moves closer to becoming a standard tool in surgical oncology, the next critical phase is obtaining approval from the FDA. A pivotal trial is set for May 2024, and Ross hopes that this advancement will grant more patients the opportunity to reach significant life milestones.
The story of Peter Ross and the SENTRY device highlights the potential of combining technology with medicine to not only enhance surgical precision but also to profoundly impact patients’ lives. As this innovation progresses through regulatory approval, it stands to change the landscape of cancer treatment and provide hope to many more individuals facing similar diagnoses.
-

 Lifestyle2 months ago
Lifestyle2 months agoWinnipeg Celebrates Culinary Creativity During Le Burger Week 2025
-

 Health2 months ago
Health2 months agoMontreal’s Groupe Marcelle Leads Canadian Cosmetic Industry Growth
-

 Science2 months ago
Science2 months agoMicrosoft Confirms U.S. Law Overrules Canadian Data Sovereignty
-

 Education2 months ago
Education2 months agoRed River College Launches New Programs to Address Industry Needs
-

 Technology2 months ago
Technology2 months agoDragon Ball: Sparking! Zero Launching on Switch and Switch 2 This November
-

 Science2 months ago
Science2 months agoTech Innovator Amandipp Singh Transforms Hiring for Disabled
-

 Technology2 weeks ago
Technology2 weeks agoDiscord Faces Serious Security Breach Affecting Millions
-

 Technology2 months ago
Technology2 months agoGoogle Pixel 10 Pro Fold Specs Unveiled Ahead of Launch
-

 Science2 months ago
Science2 months agoChina’s Wukong Spacesuit Sets New Standard for AI in Space
-

 Technology2 months ago
Technology2 months agoWorld of Warcraft Players Buzz Over 19-Quest Bee Challenge
-

 Education2 months ago
Education2 months agoAlberta Teachers’ Strike: Potential Impacts on Students and Families
-

 Business2 months ago
Business2 months agoDawson City Residents Rally Around Buy Canadian Movement
-

 Technology2 weeks ago
Technology2 weeks agoHuawei MatePad 12X Redefines Tablet Experience for Professionals
-

 Business2 months ago
Business2 months agoNew Estimates Reveal ChatGPT-5 Energy Use Could Soar
-

 Science2 months ago
Science2 months agoXi Labs Innovates with New AI Operating System Set for 2025 Launch
-

 Technology2 months ago
Technology2 months agoInnovative 140W GaN Travel Adapter Combines Power and Convenience
-

 Technology2 months ago
Technology2 months agoFuture Entertainment Launches DDoD with Gameplay Trailer Showcase
-

 Technology2 months ago
Technology2 months agoGlobal Launch of Ragnarok M: Classic Set for September 3, 2025
-

 Business2 months ago
Business2 months agoBNA Brewing to Open New Bowling Alley in Downtown Penticton
-

 Technology2 months ago
Technology2 months agoNew IDR01 Smart Ring Offers Advanced Sports Tracking for $169
-

 Education1 month ago
Education1 month agoBrandon University’s Failed $5 Million Project Sparks Oversight Review
-

 Technology2 months ago
Technology2 months agoArsanesia Unveils Smith’s Chronicles with Steam Page and Trailer
-

 Science2 months ago
Science2 months agoNew Precision Approach to Treating Depression Tailors Care to Patients
-

 Technology2 months ago
Technology2 months agoHumanoid Robots Compete in Hilarious Debut Games in Beijing