Health
New Implant Restores Blood Pressure Control for Spinal Injury Patients
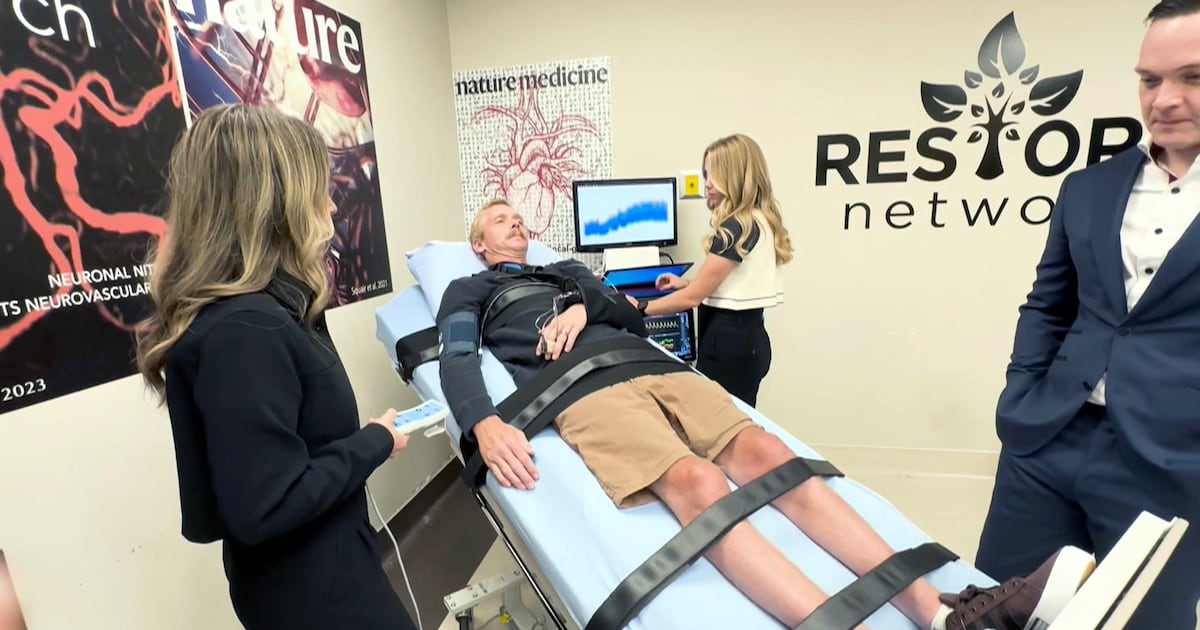
A groundbreaking implantable system has been shown to stabilize blood pressure in patients with spinal cord injuries, according to research published in the scientific journals Nature and Nature Medicine. This innovative therapy, developed by teams at multiple universities, including the University of Calgary, has the potential to significantly improve the quality of life for individuals affected by such injuries.
The study involved 14 participants across four clinical trials conducted in Canada, Switzerland, and the Netherlands. Research indicates that spinal cord injuries can disrupt the connection between the brain and the body, leading to severe blood pressure fluctuations. “Blood pressure is a profound issue after a spinal cord injury, both highs and lows,” explained Aaron Phillips, an associate dean at the University of Calgary Cumming School of Medicine, who contributed to the study.
The consequences of these fluctuations can be serious. Low blood pressure may cause fainting and fatigue, while high blood pressure increases the risk of strokes. Phillips noted that long-term blood pressure instability could also lead to cardiovascular disease.
One participant, Cody Krebs, experienced a spinal cord injury after a serious car accident in 2022 that resulted in damage to his C6 and C7 vertebrae. This injury has left Krebs unable to regulate his blood pressure effectively. “I get really lightheaded and dizzy, and my ears can start ringing,” he shared.
As part of the research trial, Krebs received an implantable device designed to help manage his blood pressure. “This device is implanted around the spinal cord and delivers a low electrical current to essentially replace the signal the brain would normally provide,” said Dr. Fady Girgis, a neurosurgeon and associate professor at the University of Calgary. He emphasized that while surgery carries risks, these devices have been used safely for years and can help patients avoid reliance on blood pressure medications.
The implant works using externally controlled electrical currents, although a new prototype allows for delivery without the need for a remote. Krebs reported significant improvements in his daily life since receiving the device. “It’s helped me get back into work because I’m not exhausted all the time,” he said. “If I have it on throughout the day, I find I can spend more time in the evenings without being tired and having to go to bed early.”
The company behind this neurostimulation system has received FDA approval to launch a pivotal trial involving approximately 20 neurorehabilitation and neurosurgical research centers across Canada, Europe, and the United States. This next phase could pave the way for wider adoption of this promising technology, potentially transforming the treatment landscape for those with spinal cord injuries.
The results of this research highlight the importance of continued innovation in medical treatments and the potential to enhance the lives of patients suffering from serious health conditions. As the medical community awaits the outcomes of the upcoming pivotal trial, the early results demonstrate a significant step forward in addressing blood pressure regulation for spinal cord injury patients.
-

 Education5 months ago
Education5 months agoBrandon University’s Failed $5 Million Project Sparks Oversight Review
-

 Science6 months ago
Science6 months agoMicrosoft Confirms U.S. Law Overrules Canadian Data Sovereignty
-

 Lifestyle5 months ago
Lifestyle5 months agoWinnipeg Celebrates Culinary Creativity During Le Burger Week 2025
-

 Health6 months ago
Health6 months agoMontreal’s Groupe Marcelle Leads Canadian Cosmetic Industry Growth
-

 Education5 months ago
Education5 months agoNew SĆIȺNEW̱ SṮEȽIṮḴEȽ Elementary Opens in Langford for 2025/2026 Year
-

 Science6 months ago
Science6 months agoTech Innovator Amandipp Singh Transforms Hiring for Disabled
-

 Technology6 months ago
Technology6 months agoDragon Ball: Sparking! Zero Launching on Switch and Switch 2 This November
-

 Business2 months ago
Business2 months agoEngineAI Unveils T800 Humanoid Robot, Setting New Industry Standards
-
 Technology3 weeks ago
Technology3 weeks agoDigg Relaunches as Founders Kevin Rose and Alexis Ohanian Join Forces
-

 Top Stories2 months ago
Top Stories2 months agoCanadiens Eye Elias Pettersson: What It Would Cost to Acquire Him
-

 Education6 months ago
Education6 months agoRed River College Launches New Programs to Address Industry Needs
-

 Business5 months ago
Business5 months agoRocket Lab Reports Strong Q2 2025 Revenue Growth and Future Plans
-

 Technology6 months ago
Technology6 months agoGoogle Pixel 10 Pro Fold Specs Unveiled Ahead of Launch
-

 Education6 months ago
Education6 months agoAlberta Teachers’ Strike: Potential Impacts on Students and Families
-

 Technology4 months ago
Technology4 months agoDiscord Faces Serious Security Breach Affecting Millions
-

 Business6 months ago
Business6 months agoBNA Brewing to Open New Bowling Alley in Downtown Penticton
-

 Science6 months ago
Science6 months agoChina’s Wukong Spacesuit Sets New Standard for AI in Space
-

 Lifestyle4 months ago
Lifestyle4 months agoCanadian Author Secures Funding to Write Book Without Financial Strain
-

 Business6 months ago
Business6 months agoNew Estimates Reveal ChatGPT-5 Energy Use Could Soar
-

 Business1 month ago
Business1 month agoNvidia and AMD CEOs Unveil AI Innovations at CES 2026
-

 Business4 months ago
Business4 months agoHydro-Québec Espionage Trial Exposes Internal Oversight Failures
-

 Top Stories4 months ago
Top Stories4 months agoPatrik Laine Struggles to Make Impact for Canadiens Early Season
-

 Business6 months ago
Business6 months agoDawson City Residents Rally Around Buy Canadian Movement
-

 Technology6 months ago
Technology6 months agoFuture Entertainment Launches DDoD with Gameplay Trailer Showcase










